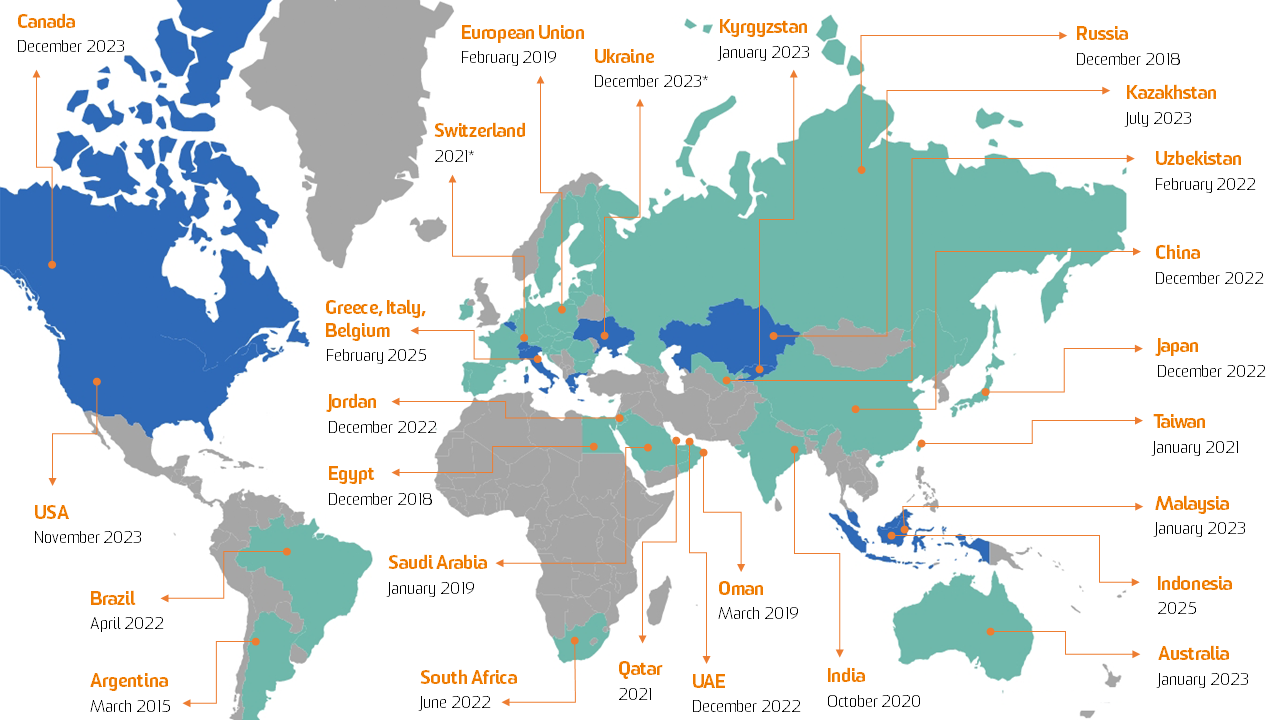
Global Serialization Landscape
Serialization is already required in countries as diverse as Argentina, Australia, Bahrain, Brazil, China, Egypt, India, Jordan, Oman, Qatar, Russia, Saudi Arabia, South Korea, Taiwan, UAE, Uzbekistan, and EU*.

The challenges of Track & Trace
The underestimated investment for implementation of serialization into the production processes has led many pharmaceutical companies to unexpected challenges as:
Lack of unified communication among business network
Additional investment in IT infrastructure
Limitation of production efficiency and lack of qualified staff
Manual work on serialization data files
Insufficient support service
The perfect fit for your serialization needs
 Easy Integration with Multiple Vendors on Level 3
Easy Integration with Multiple Vendors on Level 3
 Automatic Serialization Data Exchange
Automatic Serialization Data Exchange
 Unaffected production efficiency
Unaffected production efficiency
 Easy On-Boarding of Partners (CMOs, MAHs, Wholesales)
Easy On-Boarding of Partners (CMOs, MAHs, Wholesales)
 Automatic communication with external customer’s interface as ERP, WMS
Automatic communication with external customer’s interface as ERP, WMS
 User-friendly interface
User-friendly interface
 Data auto-publishing function
Data auto-publishing function
 Automatic conversion of serialization data files
Automatic conversion of serialization data files
 One point of contact for all serialization issues
One point of contact for all serialization issues
 All participant of the pharmaceutical chain can verify the originality and origin of the products they commercialize
All participant of the pharmaceutical chain can verify the originality and origin of the products they commercialize
They trust us for a reason



 SoftGroup provided a very clear, simple and easy-to-use technology platform and created an environment that was based on collaboration and mutual ownership. As a relatively small company, it is important to have a partner that has the capability to understand your company and the specific need of the people who need to work with the tools provided. In our experience, SoftGroup has created this environment and clearly proves to be a reliable and knowledgeable partner for our serialization needs.
SoftGroup provided a very clear, simple and easy-to-use technology platform and created an environment that was based on collaboration and mutual ownership. As a relatively small company, it is important to have a partner that has the capability to understand your company and the specific need of the people who need to work with the tools provided. In our experience, SoftGroup has created this environment and clearly proves to be a reliable and knowledgeable partner for our serialization needs.
Paul van Sprang
Sales and Marketing Director
We are on a click away.
We are determined to support the growth of your business.
Schedule a discovery call that will help us to better understand your specific case.
Click Here to Schedule Meeting





