Medical Devices
Traceability has a key role in the monitoring, supervision, and improvement of the overall quality of medical devices and in-vitro devices.
EU Regulations
In 2017 the Medical Devices Regulation (MDR) was published and marked the start of a four-year journey of transition from MDD to AIMDD. From the 26th of May 2021, the MDR will be fully applicable. The MDR affects the industries of manufacturing, distribution or procuration of medical devices.
The In vitro Diagnostic Regulation (IVDR) came into force on 25 May 2017. From 26 May 2022 new devices will have to meet the requirements of the IVDR in order to be placed in the European market. Products already certified by a Notified Body may be placed on the market until 25 May 2024 under some conditions and if the manufacturer fulfill the specific prerequisite requirements drawn in the IVDR.
What is UDI?
UDI (Unique Device Identification)
The UDI is a series of numeric or alphanumeric characters that are created through a globally accepted device identification and coding standard. It allows the unambiguous identification of a specific device on the market. The UDI is comprised of the UDI-DI and the UDI-PI.
 UDI-DI (GS1 Equivalent GTIN- Global Trade Item Number)
UDI-DI (GS1 Equivalent GTIN- Global Trade Item Number)
The UDI-DI is a unique numeric or alphanumeric code specific to a model of the device and that is also used as the ‘access key’ to the information stored in a UDI database.
 UDI- PI (GS1 Equivalent AI – Application Identifiers)
UDI- PI (GS1 Equivalent AI – Application Identifiers)
Тhe UDI-PI is a numeric or alphanumeric code that identifies the unit of device production.
The different types of UDI-PIs include a serial number, lot number, software identification, and manufacturing or expiry date, or both types of data.
UDI Implementation
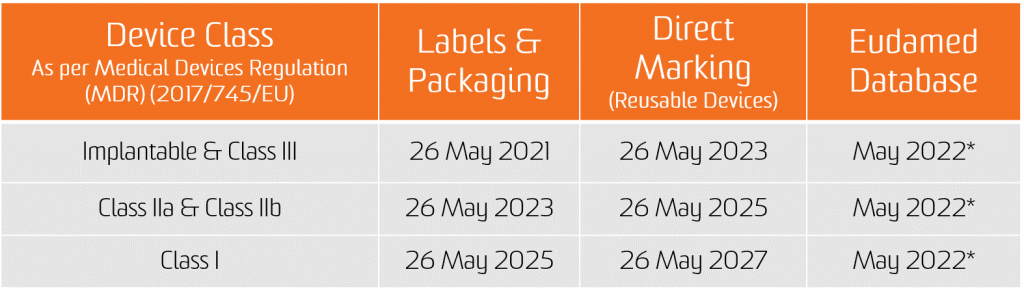
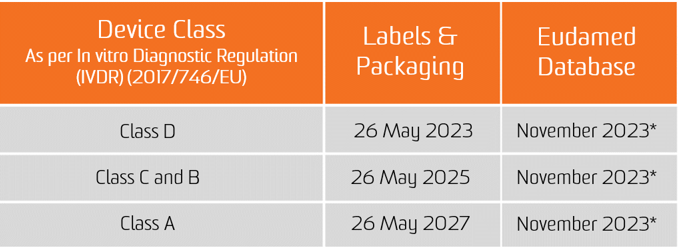
The obligation for the UDI assignment applies as from the date of application of the two new Regulations, i.e. 26 May 2021 for medical devices and 26 May 2022 for In Vitro diagnostic medical devices.
The obligation for submission of UDI data in the EUDAMED database applies from 26 November 2022 for medical devices and 26 November 2023 for in vitro diagnostic medical devices (provided that EUDAMED is fully functional before the date of application of the respective Regulation; otherwise, this obligation applies 24 months after EUDAMED has become fully functional).
However, manufacturers will be in a position to voluntarily comply with registration obligations as from 26 May 2021 for medical devices and 26 May 2022 for In Vitro diagnostic medical devices.
It shall be noted that, provided that Eudamed is fully functional, at any time after 26 May 2021 for medical devices and 26 May 2022 for In Vitro diagnostic medical devices, the full registration of devices (Article 29 of MDR and Article 26 of IVDR) remains a pre-condition for the possible registration of their relevant serious incident in Eudamed.
*CAMD issue on Nov 11, 2020 paper states that the European Commission is announcing the delay of the launch of Eudamed as a whole until May 2022 for legal reasons. The new launch date for Eudamed coincides with the implementation date for the In Vitro Diagnostic Regulation, set to take effect on May 26, 2022.
What is EUDAMED?
The new regulatory European Database on Medical Devices
Eudamed is a secure web-based portal acting as a central repository for information exchange between national Competent Authorities and the Commission in accordance with the MDR & IVDR Regulations.
The main role of Eudamed includes the following responsibilities:
Eudamed registration
UDI/Devices registration
Obtain & View Certificates from Notified Bodies
Provide clinical Investigations and performance studies data
Report vigilance and post-market surveillance
Market Surveillance Activities