Regulatory Compliance Software
Meet the Global Pharmaceutical Compliance Requirements with the industry’s most comprehensive solution on the market by SoftGroup
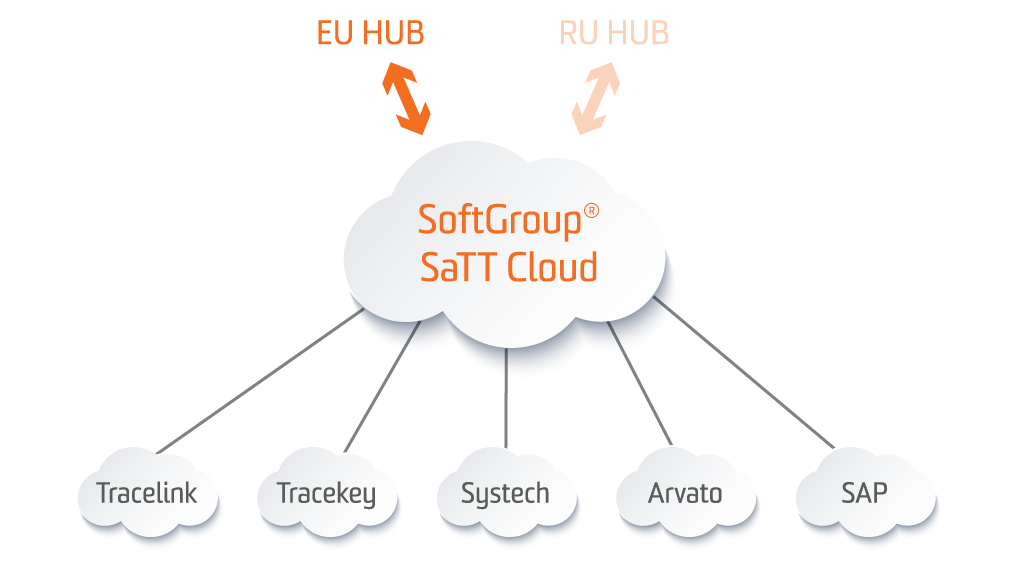
SoftGroup® SaTT EU Hub Gateway
SoftGroup® SaTT EU Hub Gateway offers an upload/update of PMD (Product Master Data) and PPD (Product Pack Data) to the EU HUB (EMVO). Via this Cloud solution, the MAH / Parallel distributor could select which serialized batch should be reported to EMVO. The system allows status verification of any reported serial number and supports all possible statuses according to the URS document designed by EMVO: active, expired, stolen, decommissioned, supplied, exported, recalled, withdrawn, destroyed, free sample, sample for NCA, locked. After transmission of data (from EMVO to the National Blueprint system), there will be a confirmation message sent to the source system. SoftGroup® SaTT EU Hub Gateway will also support all incoming messages (alerts) from EMVS.
- Product Master Data – Create/Upload/ Update Product Master Data
- Product Pack Data – Create/Upload/ Update product pack data
- Pack status update (all statuses according to the EMVO transitions rules)
- Request report
- Incoming messages (alerts and notifications) from EMVS
- User rights depending on groups membership
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
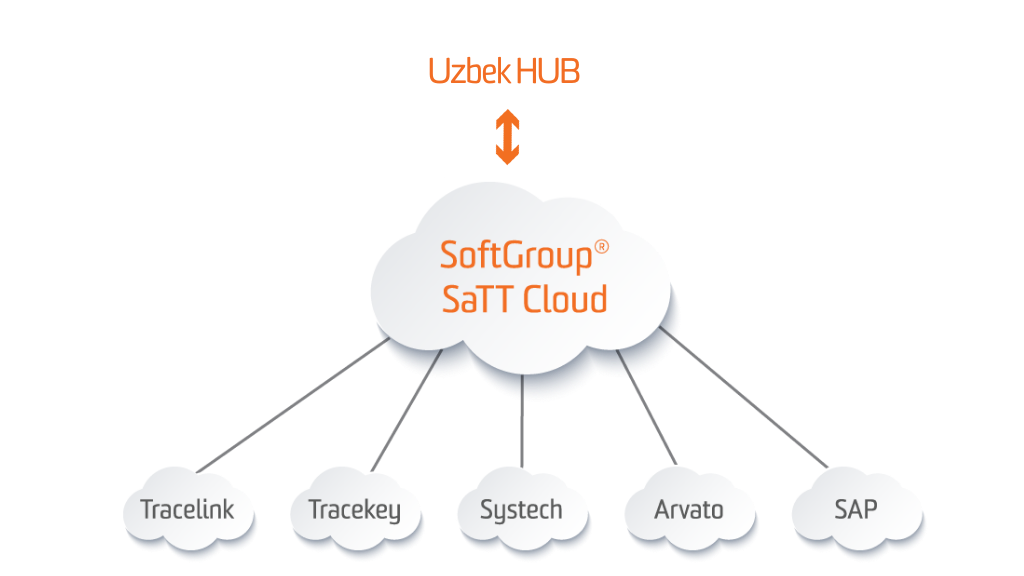
SoftGroup® SaTT Uzbek Hub Gateway
SoftGroup® SaTT Uzbek Hub Gateway provides a connection and data exchange between Uzbek National Track & Trace System – Asl belgisi and all supply-chain participants (MAHs, Manufacturers, Importers, Wholesale trade enterprises etc.). The solution allows requesting and receiving the cryptographic protection (crypto key and crypto signature for each serial number upload the final batch data) and publishing the serialization and aggregation reports concerning the Government Decree No737 “Establishment of obligatory digital labelling”. The system supports all possible statuses and functionality required by “CRPT TURON” LLC.
- Commission / Decommission unit/multi pack
- Aggregation / Deaggregation / Relabeling (Reaggregation)
- Quality shipment / QC Release
- Shipment / Receipt / Transfer
- Import / Export
- Withdrawal / Recall
- Destruction / Move Destruction
- Processing / Query result
- Notifications (receive/move order, move owner, refusal sender/receiver, retail)
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
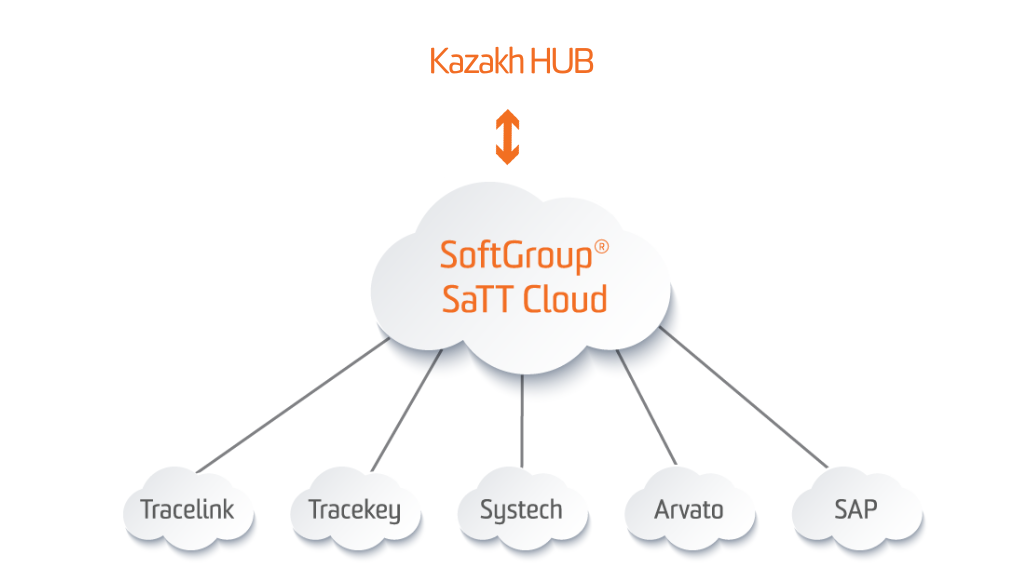
SoftGroup® SaTT Kazakh Hub Gateway
SoftGroup® SaTT Kazakh Hub Gateway provides a connection and data exchange between Kazakhstan National Track & Trace System – IS MPT and all supply-chain participants (MAHs, Manufacturers, Importers, Wholesale trade enterprises, etc.). The solution allows requesting and receiving the cryptographic protection (crypto key and crypto signature for each serial number upload the final batch data) and publishing the serialization and aggregation reports, concerning Article 7-2/12.04.2004 “About regulation of trade activity” and determining the procedure for marking and traceability of medicines in the territory of the Republic of Kazakhstan, Order No. KR DSM -11/27.01.22 “For the approval of rules for labeling of medicines and medical devices” of the Minister of Health and social development of the Republic of Kazakhstan.
The system supports all possible statuses and functionality, required by IS MPT.
- Commission / Decommission unit/multi pack
- Aggregation / Deaggregation / Relabeling (Reaggregation)
- Quality shipment / QC Release
- Shipment / Receipt / Transfer
- Import / Export
- Withdrawal / Recall
- Destruction / Move Destruction
- Processing / Query result
- Notifications (receive/move order, move owner, refusal sender/receiver, retail)
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
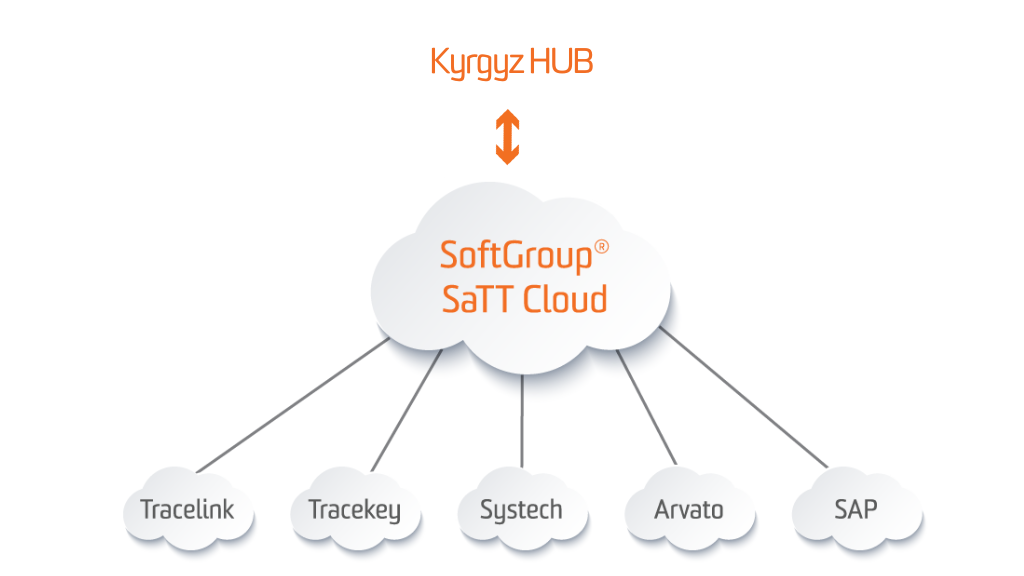
SoftGroup® SaTT Kyrgyz Hub Gateway
SoftGroup® SaTT Kyrgyz Hub Gateway provides a connection and data-exchange between Kyrgyz National Track & Trace System for pharmaceutical products and medical devices – ИС ЭБД (IS EBD) and all supply-chain participants (MAHs, Manufacturers, Importers, Wholesale trade enterprises etc.). The solution allows final batch data upload and publishing the serialization and aggregation reports, concerning Resolution No.53/9.02.2023 “Implementation of a drug traceability system in the Kyrgyz Republic“, as well as Order No. 1110/20.09.2022 “Serialization and traceability process of medicines in Kyrgyzstan” and KR-DSM -11/27.01.22 “Approval rules for marking medicinal products and medical devices” of the Minister of Health of the Republic of Kyrgyzstan.
The system supports all possible statuses and functionality, required by ИС ЭБД (IS EBD).
- Commission / Decommission unit/multi pack
- Aggregation / De-aggregation / Relabeling (Reaggregation)
- Quality shipment / QC Release
- Shipment / Receipt / Transfer
- Import / Export
- Withdrawal / Recall
- Destruction / Move Destruction
- Processing / Query result
- Notifications (receive/move order, move owner, refusal sender/receiver, retail)
Some of the functionalities will be active after their activation by Ministry of Health of the Kyrgyz Republic in the ИС ЭБД system.
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
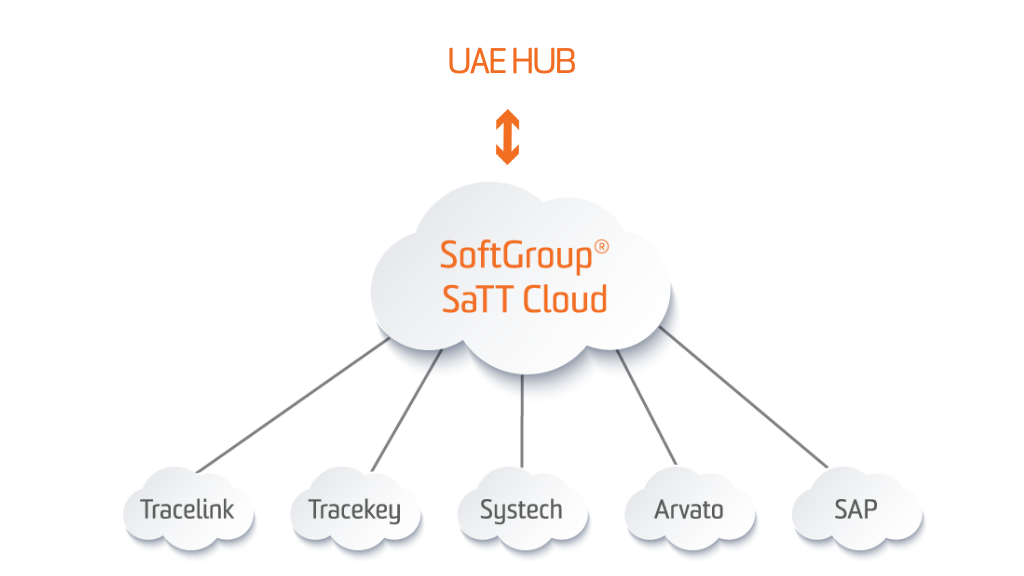
SoftGroup® SaTT UAE Hub Gateway
SoftGroup® SaTT UAE Hub Gateway provides a connection and data exchange between UAE National Track & Trace System – Tatmeen digital platform and all supply chain actors (MAHs, Manufacturers, Licensed Agent, Brand Owners, Wholesalers, Distributors, etc.). The solution will allow access to the product master data (PMD) of imported or produced drugs and uploading and publishing of the product pack data (PPD), including serial and aggregation reports of all packaging levels. SoftGroup® SaTT UAE Hub Gateway will support all incoming messages (alerts) from the Tatmeen Platform, concerning the last Ministry of Health and Prevention Resolution for tracking the pharmaceutical products and serialization guidelines.
The system will support all possible statuses and functionality, required by the UAE Ministry of Health and Prevention (“MOHAP”).
- Commissioning, Aggregation and Shipping
- Hierarchy Change: Pack (Child-Parents-Packing confirmation) & Unpack (Parents-Child-Unpacking confirmation)
- Product Transfer: Shipping, Receiving, Shipping Returns, and Receiving Returns
- Product Status Updates: Sampling, Lost, Stolen, Damaged, Dispensed, Exported, Blocked (for Inspection, Recall, Destruction), Unblocked, Recall
- Product Verification in Portal
- Requests & Receipts Confirmations, Notifications & Alerts
- Outbound & Inbound documents
- User rights depending on groups membership
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
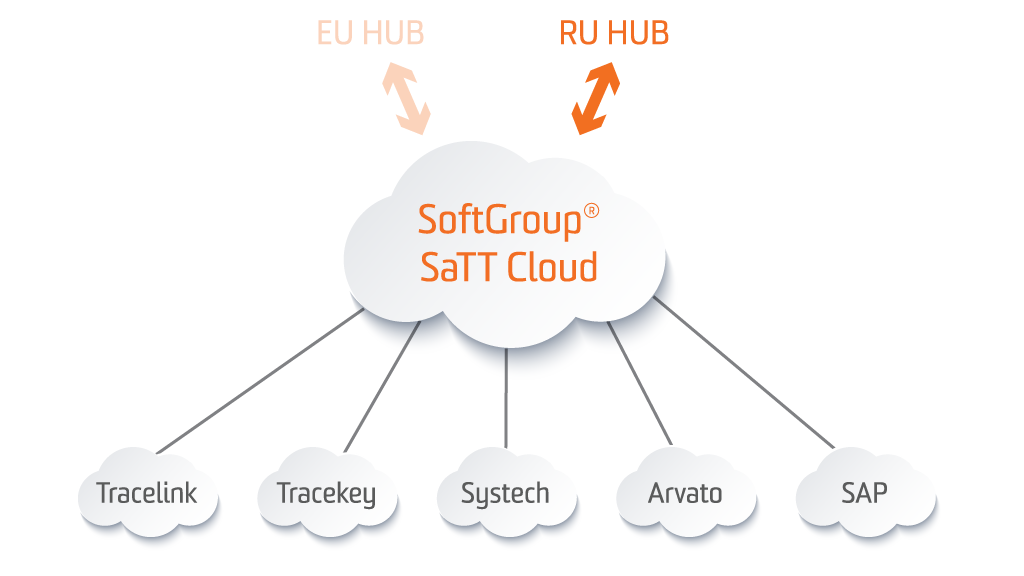
SoftGroup® SaTT RU Hub Gateway
SoftGroup® SaTT RU Hub Gateway provides a connection between the Site level and IS MDLP (Chestny ZNAK track & trace digital system) allowing an exchange of information about each product. The application allows MAHs, Manufacturers and Parallel Importers to upload the product information, to request and receive the cryptographic protection (crypto key and crypto signature for each serial number) and to report serialization and aggregation numbers (SNs & SSCCs) to IS MDLP, concerning the Decree № 1556 and № 1118. The system supports all possible statuses and functionality, required by MDLP. This data exchange is realized by using web services.
- Commission / Decommission unit/multi pack
- Aggregation / Deaggregation / Relabelling (Reaggregation)
- Quality shipment / QC Release
- Shipment / Receipt / Transfer
- Import / Export
- Withdrawal / Recall
- Destruction / Move Destruction
- Processing / Query result
- Notifications (receive/move order, move owner, refusal sender/receiver, retail)
Many thanks for your interest in our brochures. Please fill out your email and we will send you the brochure shortly.
Ready to see SoftGroup in action?
Book a demo and learn that SoftGroup can help you to:
- Generate serial number formats to meet all requirements
- Assign serial numbers and lot numbers to any product
- Data verification
- Export serialization data to subcontractors
- Generate reports and statistics
