General Overview of Russia Track and Trace System
Russia began transforming its pharma supply chain on December 29, 2017, when the President signed Federal LawNo.425-FZ. This legislation aims to streamline the quality control of pharmaceuticals, protect against counterfeit medicines, and monitor supply, demand, and expenditure.
With the regulations in Federal Law No. 425-FZ, the traceability system has been clarified and announced by Chestny Znak (Обязательная маркировка товаров – официальный сайт торговой маркировки Честный ЗНАК | Регистрация в национальной системе Честный ЗНАК (xn--80ajghhoc2aj1c8b.xn--p1ai)) to the industry authorities such as manufacturers, distributors, etc.
The main requirement of traceability systems is for companies to connect and comply with the National Track and Trace Digital System, Chestny Znak (Russian expression: Honest Sign) which is operated by the Center for Research in Perspective Technologies (CRPT) and is expected to be completely operational by 2024. By 2024, practically all consumer goods, sold, manufactured, or imported, must be marked.
The regulations cover several product categories: Medications, Antiseptic, Medical devices, Dietary Supplements, Dairy, Beverages, Bottled water, Beer and beer-based beverages, Tabacco, Wheelchairs, Light industry, Footwear, Fur, Perfumes, Tyres, Photo cameras and flashbulbs. A track & trace of sturgeon and salmon caviar, titanium metal products and bicycles and bicycle, subject to marking with the identification meansis are in experiment phase. Requirements vary by industry, but there are some common threads for all of them, such as DataMatrix codes, crypto codes, serialization, aggregation, reporting, and records management.
Russia has out in place some of the world’s strictest supply chain regulations through its National Track and Trace Digital System, known as Chestny ZNAK.
Compared to European requirements, the Russian serialization system has higher complexity as each location of the product within its supply chain in Russia should be reported to a government database.
Rules of digital marking of medicines – Track & trace for medicines
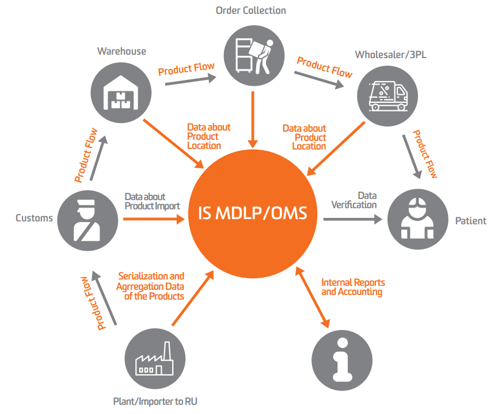
To implement the track & trace system for medicines, a dedicated information system was developed to monitor pharmaceutical product circulation (IS MDLP). The system is intended for use in the healthcare industry and is operated by the Centre for Research in Perspective Technologies (CRPT).
This digital marking process is designed to prevent the manufacture and circulation of counterfeit and falsified products.
A Data Matrix marking code is applied to secondary (retail) level of packaging. If there is no secondary packaging, the code is applied to the primary packaging.
2D Data Matrix:
- Product code/ GTIN
- Serial Number
- Verification key (i.e. Crypto key)
- Verification code (i.e. Crypto code)
Human Readable Part:
- GTIN
- Serial Number (SN)
- Lot (batch number)
- Expiration date
The marking code is requested from the operator through the emission recorder, and the system issues the code within two hours.
The price for the marking code is approved by Resolution of the Russian Government No. 577 dated 8 May 2019 and is RUB 0.5, exclusive of VAT.
Non-marked packages produced before the date of mandatory marking introduction can be sold until the expiry of the pharmaceutical products.
The track & trace system assigns a unique Data Matrix code to each product. Each code is encrypted to ensure that it cannot be massively copied or forged.
Deadlines
In accordance with the requirements of the Federal Law dd. April 12, 2010 (rev. dd. June 04, 2018) mandatory marking of medicines was introduced on January 01, 2020. According to the Decree of the Government of the Russian Federation No.1557 dd. December 14, 2018 “On the peculiarities of implementation of the track and trace system of medicines for medical use”, mandatory registration of circulation participants in the user account of MDLP IS for medicines from the list of high-cost nosologies began from July 01, 2019, marking of 7VZN medicines became mandatory from October 01, 2019.
