In this article we will give some clarifications on the use of global vs national standards for the codification of medicinal products to implement the EU Falsified Medicine Directive (EU FMD) in France.
Table of Contents
Background
In 2019, GS1 adopted rules for ensuring unique identification and integrity in its codification system, especially when third parties assign codes.
GS1 France subsequently updated its contract with the Club Inter Pharmaceutique (CIP) to align with these rules.
In December 2021, ANSM (French National Agency for the Safety of Medicines and Health Products), CIP, and GS1 France formalized a “tripartite agreement” to maintain France’s medicinal product codification system for three years (with a potential one-year extension). This agreement, along with a related decree and order from the French Ministry of Health, aims to secure the supply chain, traceability, reimbursement, and patient access to medicinal products in France, incorporating CIP and UCD codes into French law.
The codes used to identify medicinal products authorized on the French market are:
- the national number identifying each packaging called “code identifiant de présentation” (CIP code)
- and, where applicable, the number identifying the common dispensing unit (usually, the primary package level), called the “code identifiant l’unité commune de dispensation” (UCD code).
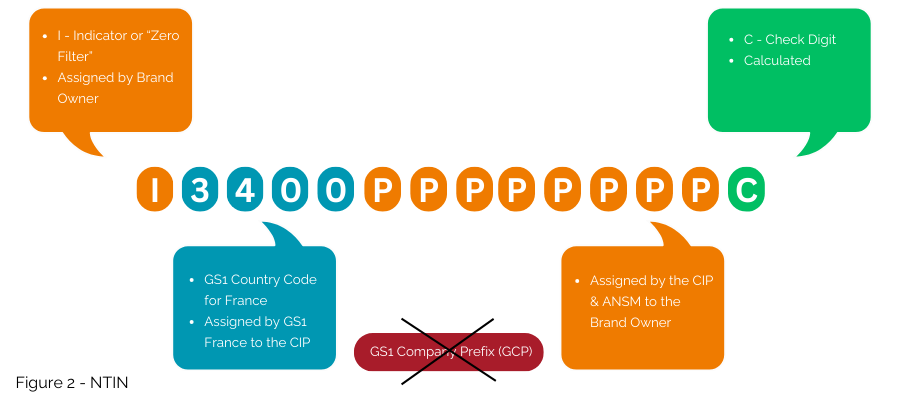
These are the current 13-character codes, with the first 4 digits being the prefix 3400 assigned by GS1 France to the CIP since 2006. This code complies with ISO/IEC 15459-3 2014 and ISO/IEC 15459-4 2014, i.e., GS1 standards recognised by ISO.
Technical considerations on a global vs national system for codification of medicinal products
The proposals for the long-term solution to be implemented in France for the codification of medicinal products include:
1. the continuation of the use of global standards based on GS1 standards as the code used for supply chain/logistic purposes (i.e. with the national number used for market autorisation and for reimbursement), either with the current National Trade Item Number (NTIN starting with 3400) or by mapping the national number to a Global Trade Item Number (GTIN) as is implemented in other EU countries.
Or
2. the transition to the use of a national standard based on the CIP code. Since May 2023, the CIP is an authorised ISO/IEC 15459 issuing agency and can generate unique identifiers starting with the prefix VIP.
Continuation of the use of global standards based on GS1 standards
First option
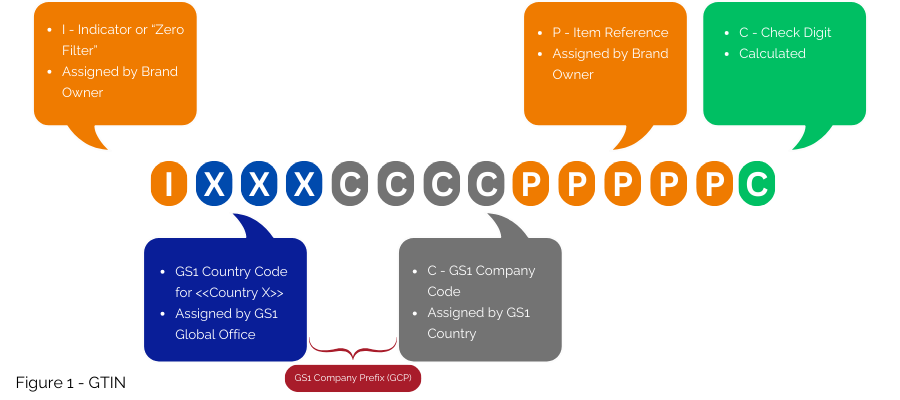
For countries like France with existing national numbering systems, GS1 offers strategies for transitioning to globally harmonized numbering. GS1 suggests using the GTIN (Figure 1) to implement the EU FMD, primarily encoding it in the GS1 DataMatrix. If a national number must be retained, it can be retrieved via a database cross-reference, without including it in the GS1 DataMatrix.
However, GS1 recognizes that in some member states, due to healthcare system management requirements or the need to transition to global standards, the national number may need to be displayed on packaging in human-readable format, not necessarily encoded in the barcode.
The proposal involves mapping the CIP code to a GTIN using a conversion table, as demonstrated in Belgium, and facilitating its use within the GS1 System. If necessary, the CIP code can be printed in human-readable format on the packaging, without encoding it in the barcode, like done in Spain today.
Second option
Second option, still compatible within the GS1 system even if not the most globally interoperable, is to embed the national number into a NTIN, instead of using a GTIN.
This is the solution currently implemented under the contract between GS1 France, the CIP and the ANSM. The GS1 prefix 3400 is licensed by GS1 France to the CIP to allow embedding the number issued by CIP and ANSM into the GTIN structure as an NTIN (Figure 2); it then enables its use within the GS1 System.
Transition to the use of a national standard based on the CIP code
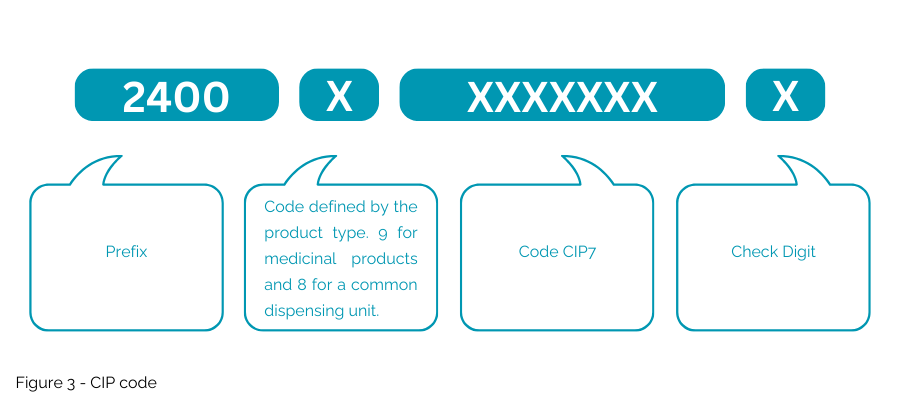
The CIP code (Figure 3) proposed is different from the current NTIN used for the French Market as the proposed “CIP code” would not be embedded into a GS1 key. This national code will only be accepted in France for specific usage and will not allow the use of multimarket packaging.
Differences between CIP code and NTIN
Here’s a comparison between the encoding of GS1 identifiers and the encoding of the proposed CIP code
1.Encoding of GS1 identifiers
- Utilizes a leading Function One character to signify GS1 DataMatrix use, invoking GS1 decoding rules.
- Include AI (01) at the beginning of the NTIN to indicate that the following characters represent a GTIN or NTIN (product code).
- Incorporate AI (17) at the beginning of the Expiration date, denoting that the following characters represent an expiration date.
- Utilize AI (10) at the beginning of the batch/lot number, indicating that the following characters represent a batch/lot number.
- Use AI (21) at the beginning of the Serial number, signifying that the following characters represent a serial number.
2. Encoding of the proposed CIP code
- Will be encoded into a generic ISO/IEC Data Matrix, necessitating a new, specific data management system.
- Use “1P” as a data delimiter at the beginning to indicate a CIP code (product code); “AI” is exclusive to the GS1 syntax.
- Incorporate “9D” as the data delimiter at the beginning for the expiration date.
- Utilize “1T” as the data delimiter at the beginning for the batch/lot number.
- Include “S” as the data delimiter at the beginning of the serial number.
Conclusion – Potential impact of the implementation of a new codification of medicinal products
The labels and packaging of medicinal products supplied to France will need to be revised in order to carry the generic ISO Data Matrix (Figure 4) encoding the CIP code and other data attributes. The GS1 DataMatrix will continue to be used in other EU countries (Figure 5).
(Image from Discussion paper on the use of global vs national standards for the condification of medicinal products to implement the EU Falsified Medicine Directive in France)
The proposed changes in encoding medicinal product data, specifically the shift from GS1 standards to a unique national coding system in France, pose significant challenges. Existing scanning, decoding, and processing systems will be incapable of automatically interpreting data within the new Data Matrix without custom upgrades. This change not only affects manufacturers but also could cause turbulence in the entire supply chain, impacting hospitals and pharmacies that are already delayed in implementing the EU FMD.
This would undermine the interoperability within the French system and between the French system and the other EU systems, as well as be a threat to the access to medicinal products in France and the competitiveness of the local industry.
Source: GS1 | Discussion paper on the use of global vs national standards for the codification of medicinal products to implement the EU Falsified Medicine Directive in France